ALPHANATE clinical data
The efficacy of ALPHANATE was demonstrated to be good or excellent in the majority of procedures in patients with VWD1
- ALPHANATE is effective in preventing excessive perioperative bleeding in patients with von Willebrand disease (VWD). ALPHANATE demonstrated good or excellent efficacy in the majority of procedures, including a wide range of major and minor surgical and invasive procedures
- ALPHANATE is indicated for surgical and/or invasive procedures in adult and pediatric patients with VWD in whom desmopressin (DDAVP) is either ineffective or contraindicated. It is not indicated for patients with severe VWD (Type 3) undergoing major surgery
In a prospective study, ALPHANATE was used for prophylaxis during surgery or invasive procedures in patients with VWD1
Number of infusions of ALPHANATE over the perioperative period*
| Number of patients | 37 |
| Number of surgical procedures | 59 |
| Median number of infusions per surgical procedure (1-18) | 4 |
| MEDIAN DOSAGE IU/ VWF:RCO/KG | |
| Infusion #1 (range) | 59.9 (19.8-75.1) |
| Infusion ≥#2 (combined range) | 40.0 (4.5-75.1) |
*Represents total number of patients, procedures, and medians for patients treated with both ALPHANATE (A-SD) (n=21) and ALPHANATE (A-SD/HT) (n=18) in surgery. ALPHANATE, Solvent Detergent, Non–Heat-Treated (A-SD), is the previous formulation and ALPHANATE, Solvent Detergent/Heat-Treated (A-SD/HT), is the current formulation. Both products are biochemically equivalent and demonstrate similar in vivo pharmacokinetic profiles. Both products are also similarly effective for the treatment of bleeding episodes and provide adequate hemostasis for surgical and invasive procedures, even in the absence of bleeding time correction, in subjects with moderate and severe VWD.
The most frequent adverse drug reactions reported with ALPHANATE in >1% of infusions were pruritus, headache, back pain, paresthesia, respiratory distress, facial edema, pain, rash and chills.
Physicians confirmed the investigator ratings of ALPHANATE from the prospective study
92% of all procedures were rated as good or excellent by the investigator
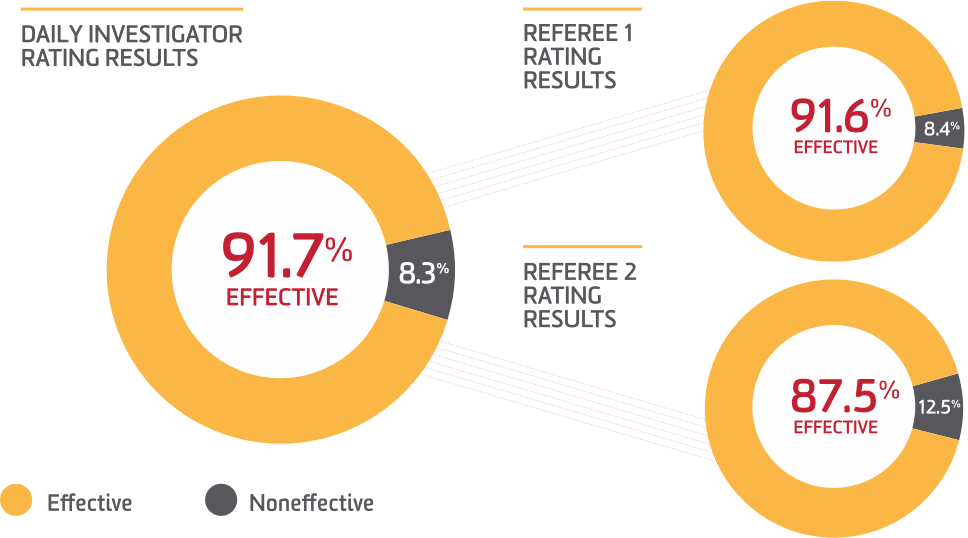
Hemostasis of ALPHANATE patients is comparable to individuals without known bleeding disorders1
- ALPHANATE received an overall rating of good or excellent in 92% of procedures, meaning hemostasis was comparable/slightly inferior to that expected for individuals without known bleeding disorders
- There were 92% and 88% success rates for patients treated with ALPHANATE undergoing surgery, as determined by 2 referees
An established, proven safety and tolerability profile for ALPHANATE1
| Number of patients | Number of infusions | Patients with adverse events (AEs) possibly, probably, or definitely related to drug | Infusions with AEs possibly, probably, or definitely related to drug | Type of AEs | Pediatric patients |
|---|---|---|---|---|---|
| 36 | 204 | 13.9% | 6.9% | All mild except1 moderate (pruritus) | 1 AE (pain) in4 patients |
One incident of pulmonary embolus was reported that was considered to have a possible relationship to the product. This subject received repeated doses of 60 IU VWF:RCo/kg body weight and the FVIII:C level achieved was 290%.
Retrospective study supports prophylactic use of ALPHANATE in patients with VWD1,2
- In a retrospective study, ALPHANATE was also used for prophylaxis during surgery or invasive procedures and demonstrated excellent to good efficacy in the majority of the procedures†
- The mean number of infusions for 39 patients undergoing 61 surgical procedures was 5.9; the median number of infusions per surgical procedure was 3 (range 1-27)
92% of all outcomes were rated as good or excellent by the referees2
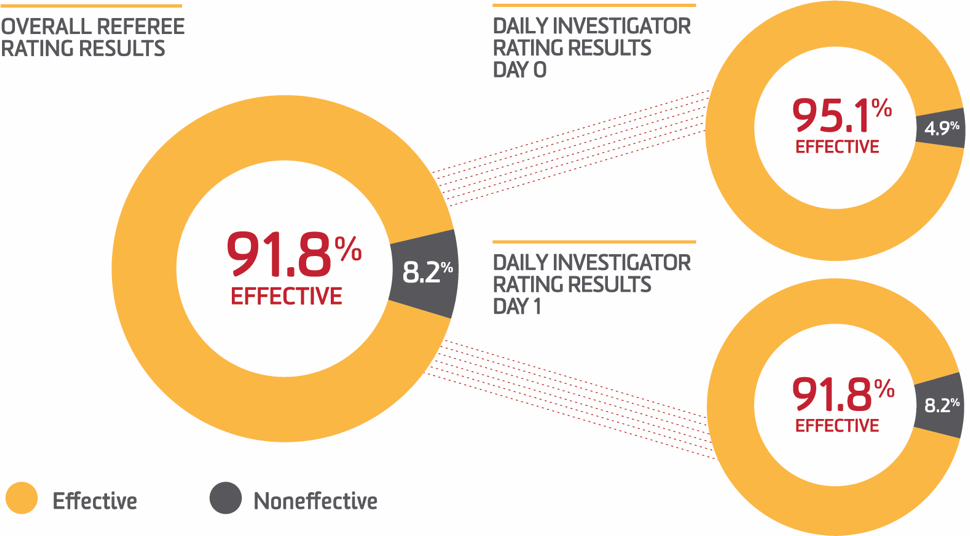
Comparable hemostasis to individuals without known bleeding disorders2
- ALPHANATE received a daily investigator rating of good or excellent in 92% of procedures, meaning hemostasis was comparable/slightly inferior to that expected for individuals without known bleeding disorders2
- Physician referees were in agreement with investigators in a retrospective ALPHANATE efficacy and safety study, whereby 92% of all outcomes were rated as good or excellent by the referees
†Results obtained from a retrospective evaluation of 61 surgical or invasive procedures (type 1: 7 major and 15 minor; type 2: 4 major and 19 minor; type 3: 1 major and 15 minor).
ALPHANATE adverse events were mild to moderate1
| Number of patients | Number of infusions/procedures | Patients with adverse drug reactions (ADRs) with possible or stronger causal relationship to study drug | Type of ADRs | Pediatric patients |
|---|---|---|---|---|
| 39 | 5.9 | 7.7% | 4 mild 2 moderate | No ADRs in 5 patients |
| Number of patients | 39 |
| Number of infusions/procedures | 5.9 |
| Patients with adverse drug reactions (ADRs) with possible or stronger causal relationship to study drug | 7.7% |
| Type of ADRs | 4 mild 2 moderate |
| Pediatric patients | No ADRs in 5 patients |
Indication
ALPHANATE® (antihemophilic factor/von Willebrand factor complex [human]) is indicated for:
- Control and prevention of bleeding episodes and perioperative management in adult and pediatric patients with factor VIII (FVIII) deficiency due to hemophilia A.
- Surgical and/or invasive procedures in adult and pediatric patients with von Willebrand disease (VWD) in whom desmopressin (DDAVP) is either ineffective or contraindicated. It is not indicated for patients with severe VWD (type 3) undergoing major surgery.
Important Safety Information
ALPHANATE is contraindicated in patients who have manifested life-threatening immediate hypersensitivity reactions, including anaphylaxis, to the product or its components.
Anaphylaxis and severe hypersensitivity reactions are possible with ALPHANATE. Discontinue use of ALPHANATE if hypersensitivity symptoms occur, and initiate appropriate treatment.
Development of procoagulant activity-neutralizing antibodies (inhibitors) has been detected in patients receiving FVIII-containing products. Carefully monitor patients treated with AHF products for the development of FVIII inhibitors by appropriate clinical observations and laboratory tests.
Thromboembolic events have been reported with AHF/VWF complex (human) in VWD patients, especially in the setting of known risk factors.
Intravascular hemolysis may occur with infusion of large doses of AHF/VWF complex (human).
Rapid administration of a FVIII concentrate may result in vasomotor reactions.
Because ALPHANATE is made from human plasma, it may carry a risk of transmitting infectious agents, eg, viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent, despite steps designed to reduce this risk.
Monitor for development of FVIII and VWF inhibitors. Perform appropriate assays to determine if FVIII and/or VWF inhibitor(s) are present if bleeding is not controlled with expected dose of ALPHANATE.
The most frequent adverse drug reactions reported with ALPHANATE in >1% of infusions were pruritus, headache, back pain, paresthesia, respiratory distress, facial edema, pain, rash, and chills.
Please see full Prescribing Information for ALPHANATE.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1.800.FDA.1088.
References:
- ALPHANATE® (antihemophilic factor/von Willebrand factor complex [human]) Prescribing Information. Grifols.
- Rivard GE, Aledort L. Haemophilia. 2008;14(2):271-275.


