ALPHANATE is a human plasma-derived treatment for patients with hemophilia A or von Willebrand disease1
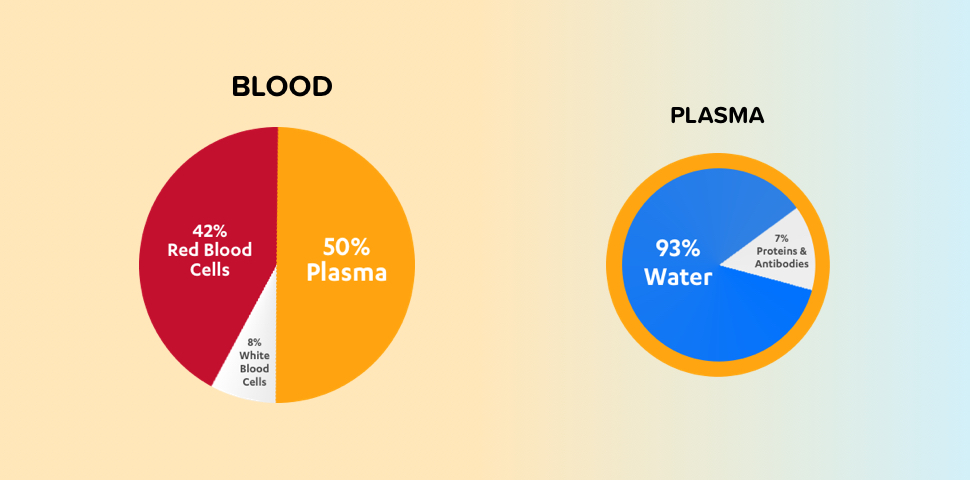
Plasma and clotting factors in your blood
Plasma is a clear, straw-colored liquid component of blood composed of mostly water, salts, and proteins. Plasma contains proteins needed for clotting blood and defending the body against infection. These proteins include factor VIII (FVIII) and von Willebrand factor (VWF).
Clotting factors are proteins present in plasma that work together to stop bleeding. Hemophilia is an inherited bleeding disorder caused by low levels or a complete lack of certain clotting factors. Individuals with hemophilia A, the most common type of hemophilia, do not have enough FVIII in their plasma. Von Willebrand disease (VWD) is a genetic bleeding disorder caused by missing or defective VWF.2,3
What is ALPHANATE?
ALPHANATE is a natural, human plasma-derived factor complex that contains both FVIII and VWF. It is produced through a manufacturing process specifically designed to preserve FVIII and VWF together, the way they are found naturally in the body.1
ALPHANATE is an FDA-approved human plasma-derived FVIII/VWF treatment for adults and pediatric patients with hemophilia A or VWD.1

How ALPHANATE works
ALPHANATE is a human plasma-derived FVIII/VWF complex, which means ALPHANATE contains both FVIII and VWF. In the body, FVIII and VWF naturally travel together to provide a foundation of natural protection to build on.1
Indication
ALPHANATE® (antihemophilic factor/von Willebrand factor complex [human]) is indicated for:
- Control and prevention of bleeding episodes and perioperative management in adult and pediatric patients with factor VIII (FVIII) deficiency due to hemophilia A.
- Surgical and/or invasive procedures in adult and pediatric patients with von Willebrand disease (VWD) in whom desmopressin (DDAVP) is either ineffective or contraindicated. It is not indicated for patients with severe VWD (type 3) undergoing major surgery.
Important Safety Information
ALPHANATE is contraindicated in patients who have manifested life-threatening immediate hypersensitivity reactions, including anaphylaxis, to the product or its components.
Anaphylaxis and severe hypersensitivity reactions are possible with ALPHANATE. Discontinue use of ALPHANATE if hypersensitivity symptoms occur, and initiate appropriate treatment.
Development of procoagulant activity-neutralizing antibodies (inhibitors) has been detected in patients receiving FVIII-containing products. Carefully monitor patients treated with AHF products for the development of FVIII inhibitors by appropriate clinical observations and laboratory tests.
Thromboembolic events have been reported with AHF/VWF complex (human) in VWD patients, especially in the setting of known risk factors.
Intravascular hemolysis may occur with infusion of large doses of AHF/VWF complex (human).
Rapid administration of a FVIII concentrate may result in vasomotor reactions.
Because ALPHANATE is made from human plasma, it may carry a risk of transmitting infectious agents, eg, viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent, despite steps designed to reduce this risk.
Monitor for development of FVIII and VWF inhibitors. Perform appropriate assays to determine if FVIII and/or VWF inhibitor(s) are present if bleeding is not controlled with expected dose of ALPHANATE.
The most frequent adverse drug reactions reported with ALPHANATE in >1% of infusions were pruritus, headache, back pain, paresthesia, respiratory distress, facial edema, pain, rash, and chills.
Please see full Prescribing Information for ALPHANATE.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1.800.FDA.1088.
References:
- ALPHANATE® (antihemophilic factor/von Willebrand factor complex [human]) Prescribing Information. Grifols.
- Hemophilia A (factor VIII deficiency). National Hemophilia Foundation Website. https://www.hemophilia.org/bleeding-disorders-a-z/types/hemophilia-a. Accessed August 31, 2022.
- Von Willebrand Disease. National Hemophilia Foundation website. https://www.hemophilia.org/bleeding-disorders-a-z/types/von-willebrand-disease. Accessed August 31, 2022.
- Federici AB, Mannucci PM. Ann Med. 2007;39(5):346-358.
- Franchini M, Lippi G. Thromb Haemost. 2010;104:931-940.
- Lacroix-Desmazes S, et al. Blood. 2008;112(2):240-249.


