ALPHANATE is a human plasma-derived factor complex that contains both factor VIII (FVIII) and von Willebrand factor (VWF).1
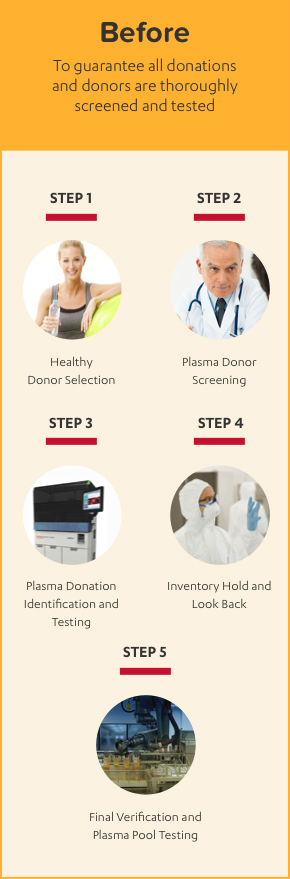
ALPHANATE IS MADE WITH A 10-STEP PLASMA SAFETY PROCESS
ALPHANATE is manufactured using 10 steps to safety with full traceability through the entire process from donor to patient.


![ALPHANATE PLASMA SAFTEY AND PURITY PROCESS]](/documents/729928/730222/10Steps_Images_Desktop_After_Desktop.jpg/057649a2-0113-4856-9d8d-910694985644?t=1497366149000)
Because ALPHANATE is made from human plasma, it may carry a risk of transmitting infectious agents, eg, viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent, despite steps designed to reduce this risk.
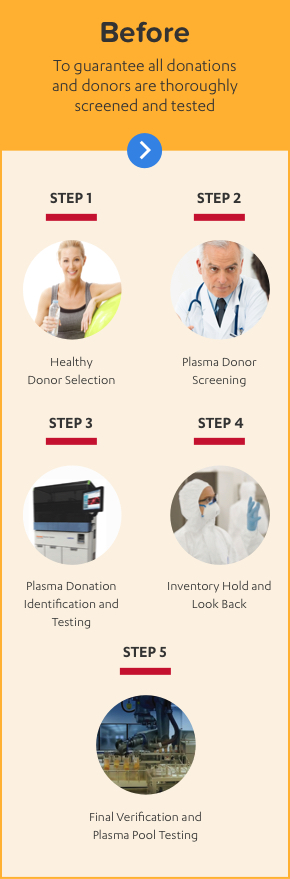
ALPHANATE IS MADE WITH A 10-STEP PLASMA SAFETY PROCESS
ALPHANATE is manufactured using 10 steps to safety with full traceability through the entire process from donor to patient.


![ALPHANATE PLASMA SAFTEY AND PURITY PROCESS]](/documents/729928/730201/2.0_Purity_and_Safety_After_Desktop.jpg/6f47e9df-636a-4748-8cc9-a03872940f15?t=1495629209000)
Because ALPHANATE is made from human plasma, it may carry a risk of transmitting infectious agents, eg, viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent, despite steps designed to reduce this risk.
ALPHANATE PATIENT SUPPORT AND RESOURCES

Factors for Health
A comprehensive patient support and assistance program.

Talk to your doctor
The Doctor Discussion Guide will give you information to help you ask key questions and have an informative conversation with your doctor.

Grifols Gear travel kit
The Grifols Gear travel kit makes infusions easier when you are on the go.

Hemophilia A & VWD Resources
Check out these helpful links for people with bleeding disorders.
LIVING WITH HEMOPHILIA A
Indication
ALPHANATE® (antihemophilic factor/von Willebrand factor complex [human]) is indicated for:
- Control and prevention of bleeding episodes and perioperative management in adult and pediatric patients with factor VIII (FVIII) deficiency due to hemophilia A.
- Surgical and/or invasive procedures in adult and pediatric patients with von Willebrand disease (VWD) in whom desmopressin (DDAVP) is either ineffective or contraindicated. It is not indicated for patients with severe VWD (type 3) undergoing major surgery.
Important Safety Information
ALPHANATE is contraindicated in patients who have manifested life-threatening immediate hypersensitivity reactions, including anaphylaxis, to the product or its components.
Anaphylaxis and severe hypersensitivity reactions are possible with ALPHANATE. Discontinue use of ALPHANATE if hypersensitivity symptoms occur, and initiate appropriate treatment.
Development of procoagulant activity-neutralizing antibodies (inhibitors) has been detected in patients receiving FVIII-containing products. Carefully monitor patients treated with AHF products for the development of FVIII inhibitors by appropriate clinical observations and laboratory tests.
Thromboembolic events have been reported with AHF/VWF complex (human) in VWD patients, especially in the setting of known risk factors.
Intravascular hemolysis may occur with infusion of large doses of AHF/VWF complex (human).
Rapid administration of a FVIII concentrate may result in vasomotor reactions.
Because ALPHANATE is made from human plasma, it may carry a risk of transmitting infectious agents, eg, viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent, despite steps designed to reduce this risk.
Monitor for development of FVIII and VWF inhibitors. Perform appropriate assays to determine if FVIII and/or VWF inhibitor(s) are present if bleeding is not controlled with expected dose of ALPHANATE.
The most frequent adverse drug reactions reported with ALPHANATE in >1% of infusions were pruritus, headache, back pain, paresthesia, respiratory distress, facial edema, pain, rash, and chills.
Please see full Prescribing Information for ALPHANATE.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1.800.FDA.1088.
Reference:
- ALPHANATE® (antihemophilic factor/von Willebrand factor complex [human]) Prescribing Information. Grifols.